
(in other words we reduced 100% to decimal form 1. We will let 6Li = x and 7 Li = 1-x we use 1 – x instead of 100 – x because the small number is easier to work with. Thus, Avogadros number has been measured to be 6.022141×10²³.

Or, a little easier to understand, Avogadros number is the ratio of 1 gram to 1 atomic mass unit but with the atomic mass unit expressed in grams. Since I don’t know what the percentage are, I will have to use variables.ġ00% of Lithium is determined by these two naturally occurring isotopes. Avogadros number is the ratio of the mass of 12 grams of carbon-12 to the mass of 1 atom of carbon-12 measured in grams. Determine the percent abundance of each isotope.Īw = + + Ħ.94 = + The atomic mass of lithium is 6.94, the naturally occurring isotopes are 6Li = 6.015121 amu, and 7Li = 7.016003 amu. Molecular formulas may not be unique the same types and numbers of atoms can bond to each other. Atomic number (Z) of an element number of protons number of electrons.
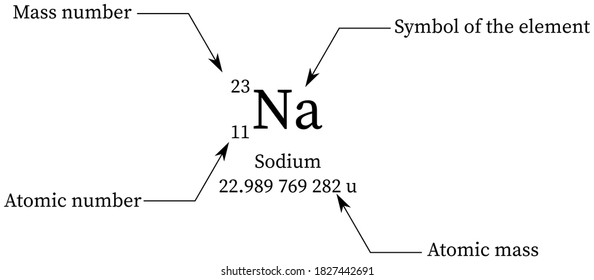
Since the atom is electrically neutral so the number of protons and number of electrons are equal. Atomic mass for Cu = 63.546Ħ3.546 = + Ħ5Cu = 1 – x = 1 – 0.6916 = 0.3084 x 100% = 30.84% The number of neutrons is equal to the difference between the mass number of the atom (M) and the atomic number (Z). The molecular formula tells the number of each type of atom. The number of protons in the atom of an element is known as atomic number. What are the percent abundances of the isotopes? Since the overall atomic weight for copper is not given in the problem, you must look it up in the periodic table to work this solution. If you look in the periodic table you will be able to check that our answer is correct!ģVerify that the atomic mass of magnesium is 24.31, given the followingĪtomic mass= + + ĭetermining the percent abundance of each isotope from atomic mass.Ĭopper exists as two isotopes: 63Cu (62.9298 amu) and 65Cu (64.9278 amu).

10.81amu so, the atomic weight of B = 10.81amu To determine relative atomic mass, we simply multiply each isotopic mass by its abundance, add all the values together and divide the total value by 100 percent.


 0 kommentar(er)
0 kommentar(er)
